⇧ (VIDEO) You might also like this affiliate content
Thirteen years after the nuclear disaster in Fukushima, Japan, researchers have for the first time taken direct images of radioactive cesium atoms in environmental samples and possibly from one or more reactors at the Fukushima Daiichi plant. The breakthrough was made by analyzing radioactive materials called “pollucites”, which form inside damaged reactors. The results provide valuable data to help address the ongoing challenges of nuclear waste management.
In March 2011, a nuclear disaster occurred at the Fukushima Daiichi Power Plant (FDNPP), following a tsunami caused by a magnitude 9.1 earthquake. A wave of 15 meters washed over the power plant, disabling reactor cooling systems, emergency power and radioactive fuel storage pools. These failures led to the melting of the core of three reactors, as well as to overheating of the deactivation pool of the fourth reactor.
The accident resulted in radioactive contamination of a huge area covering more than a million square kilometers, stretching from the part of the sea area next to the power plant to the center of Tokyo. It is the second largest nuclear disaster in history, with a level of severity similar to Chernobyl.
Since the accident, numerous decontamination efforts have been made. However, despite reductions in radiological doses in most contaminated areas, concerns remain about the persistence and behavior of radioactive cesium (Cs) in the environment. This element currently dominates contaminated areas due to its half-life of 30.1 years.
In order to optimize the management of radioactive Cs, research is focused on understanding the properties of fuel residues (a mixture of melted nuclear fuel and structural materials) found inside damaged reactors. A large amount of radioactive Cs accumulates especially in the form of particles in the latter. Called Cs-rich microparticles (CsMP), they have a composition similar to glass and are poorly soluble. They form at the bottom of the reactor when molten fuel interacts with concrete.
After formation, CsMP leaves the reactor and spreads in the surrounding environment. Therefore, it is necessary to carefully collect and dispose of them. However, uncertainties remain regarding the physicochemical properties of these particles, making recovery attempts difficult.
As part of his new study, recently published in
Journal of Hazardous Materials, an international team of researchers has just significantly advanced our understanding of CsMPs by showing for the first time the radioactive Cs atoms they contain. ” We confidently demonstrate a new occurrence of Cs associated with materials released by FDNPP reactors (Fukushima),” explains ua communicated one of the authors of the study, Gareth Law, from the University of Helsinki (Finland).
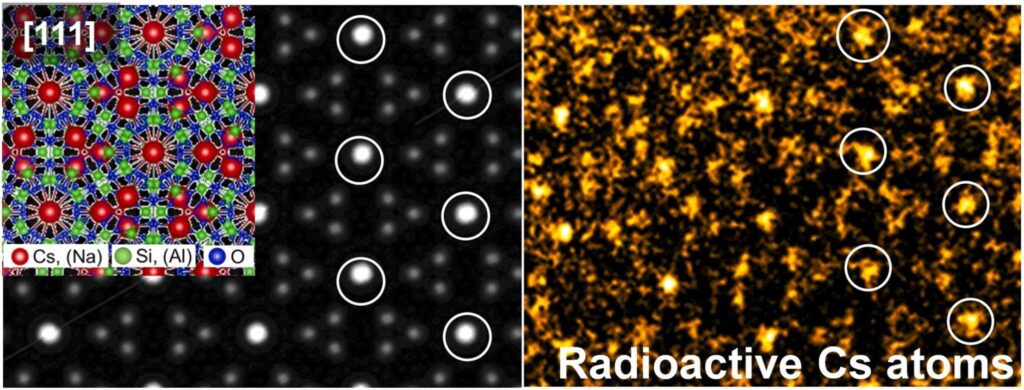
Left: modeling the structure of the half cell. Right: Direct image of Cs atoms appearing as bright spots (circled in the image). About half of the Cs atoms in the structure are radioactive. © Kanako Miyazaki et al.
Behavior different from other radioactive cesium fallout
In order to assess the extent and mechanisms of coupling, the researchers previously carried out a more or less detailed characterization of CsMP. However, direct imaging at the atomic level has not been performed so far. ” This means that we lack complete information about the chemical form of Cs in fuel particles and debris. », Law on Assessments.
Indeed, although Cs embeds these particles in relatively high concentrations, they remain too weak for atomic imaging based on electron microscopy. On the other hand, when Cs is detected at high enough levels, the electron beams used for imaging damage the samples.
To solve this problem, the research team relied on high-resolution, wide-angle, annular dark-field scanning electron microscopy (HR-HAADF-STEM). Analyzing the CsMPs, they discovered that they are coated with a zeolite-like mineral (crystals composed of a microporous aluminosilicate framework) called pollucite. While semi-cytes in nature are generally rich in aluminium, those in CsMPs are rich in iron.
” The contamination in CsMP was clearly different from that in nature, indicating that it originated in the reactors. (AND), Since we knew that most of the Cs in CsMPs comes from fission, we thought that half-cell analysis might provide the first direct images of radioactive Cs atoms “, says Law. Note that zeolites become amorphous when exposed to an electron beam, but this damage is related to the composition of the mineral. Certain inclusions of polcite thus retained their stability, which enabled the display of Cs atoms.
The obtained images revealed that Cs is heterogeneously distributed in CsMP residues. The presence of polcite indicates that Cs reacted with silica-rich materials during reactor melting, possibly by volatilization or condensation. The impurities contained Cs in the amount of 27 to 36% of the total weight.
According to Bernd Grambow of the University of Nantes, “we should now start to consider the behavior of Cs pollucite in the environment and its potential impacts. It probably behaves differently from other forms of Cs spillover documented so far.” Effects on human and animal health should also be assessed, for example by analyzing the chemical reactivity of the platelets with body fluids. According to experts, these effects could be radically different from what was observed before.

